Owners of patent RU 2567294:
The invention relates to the modification of alkaline caprolactam production waste (ShSPK) for use as an independent product or as part of solutions and mixtures used at low temperatures (below 0°C), for example, as: antifreeze, de-icer, de-icing agent, anti-freezing agent , adhesion, dusting and blowing, anti-caking agent, preventive lubrication, etc. The method of modifying the alkaline wastewater of caprolactam production is to treat it with an acid or a mixture thereof or an aqueous solution of an acid or a mixture thereof to a pH value of 4-9. The technical result is the creation of a technologically simple, inexpensive method for modifying ShchSPK, as well as a solution for use at low temperatures with high performance characteristics: low pour point down to minus 35-70 ° C and low viscosity during long-term operation at low temperatures and stability of properties under long-term conditions exposure to low temperatures. 2 n. and 7 salary files, 1 table.
Field of invention
The invention relates to the pre-treatment (modification) of alkaline wastewater from the production of caprolactam (ShSPK) for use as an independent product or as part of solutions and mixtures used at low temperatures (below 0°C), for example, as: antifreeze, deicer, deicing agent , anti-freezing, sticking, dusting and blowing agents, anti-caking agents, preventative lubricants, etc.
State of the art
ShchSPK is used in the construction industry and the building materials industry as a plasticizing-air-entraining additive in concrete, reinforced concrete, mortars, in the production of cement, porcelain, gypsum binder, fire supplies (refractories), expanded clay gravel, brick, for liquefying the initial raw material mixtures of clinker mineralization during cement production, in oil production - to increase oil recovery, as well as independently and as part of solutions and mixtures in deicers, deicing agents, in preventive agents for the treatment of transport and mining equipment and for the treatment of bulk and/or wet materials such as coal, ore , sand, etc. to prevent freezing, freezing, dusting and blowing out.
Alkaline caprolactam production waste (CAE), a large-scale waste product from caprolactam production, is an aqueous solution of sodium salts (mostly sodium adipate) of the acidic by-products of air oxidation of cyclohexane.
ShchSPK is a liquid from brown to dark brown, opaque, without visible mechanical impurities.
Composition of ShchSPK produced by OJSC KuibyshevAzot (in wt.%) and properties:
Composition of ShchSPK (ShchKPK) produced by KemerovoAzot OJSC (in wt.%) and properties:
It is generally accepted that the use of alkaline waste from caprolactam production is due to the low viscosity value at low temperatures and low pour point (up to -35°C), as well as the large available volumes of the raw material base.
Such properties of ACHSPK are determined by the content in its composition of sodium salts of low molecular weight carboxylic acids (mostly sodium adipate), which lower the pour point of aqueous solutions and modify crystal formation (the effect of ice melting).
From RF patent No. 2280666, publ. 07/27/2006, a means for combating ice is known, which is an aqueous solution of ShchSPK with a concentration of 30-100%.
From the RF author's certificate No. 1816786, publ. 05/07/1988, a solution (emulsion) is known, used for dust removal and blowing of bulk materials in the mining industry, containing a 0.1-0.3% solution of alkaline waste from the production of caprolactam.
From RF patent No. 2486223, publ. 06/27/2013, a solution is known for coating the metal surfaces of cars and other mining and transport equipment against freezing and sticking to them of overburden rocks, coal, ore, limestone and other wet bulk materials, containing an alkaline drain from the production of caprolactam and a stabilizing additive that prevents delamination and reduces pour point, which is used as alcohols or salts.
The proposed product solves the technical problem of expanding the raw material base through the use of caprolactam production waste; a decrease in the pour point is achieved by introducing a stabilizing additive into the composition. In addition, a decrease in viscosity at low temperatures helps to reduce energy costs when treating with a prophylactic agent and obtain a more uniform coating layer.
The closest to the stated solution is the one known from copyright certificate No. 1680750, publ. 09/30/1991, a solution used as a means of preventing solid fuels from blowing out and freezing during transportation, which contains an alkaline waste from caprolactam production and an aqueous acidic waste from caprolactam production. The solution according to the description of the invention has high resistance to delamination. However, it is characterized by a pour point of the order of (-25)-(-34) °C, which is not enough for processing wet bulk materials in winter. When the bulk materials treated with the solution are kept for 5 hours at temperatures (-25)-(-35)°C, freezing of the material is observed, and at a temperature of minus 34°C, separation (precipitation) is observed in the solution. Moreover, an increase in the acidity of the solution to pH=6.5 leads to an increase in the pour point of the solution, and an increase in alkalinity to pH=9.5 leads to an increase in viscosity, and at minus 34°C, precipitation.
The main significant disadvantage of ShchSPK and known solutions based on ShchSPK is that when they are thermostatted for a long time at low temperatures (maintenance at temperatures below minus 20°C for at least 3 hours), a sharp increase in viscosity occurs, precipitation occurs (in solutions of ShchSPK) and As a consequence, solidification of ShchSPK or solutions with ShchSPK occurs at a temperature significantly higher than the declared nominal pour point.
Application in solutions based on ACHSPK used at low temperatures (below 0°C) (antifreeze, de-icers, de-icing agents, anti-freezing, sticking, dusting and blowing agents), components that lower the pour point of aqueous solutions, such as monohydric alcohols, polyhydric alcohols, alkylene glycols, alkylene glycol ethers, salts of organic and/or inorganic alkali metal acids, do not significantly change the indicated properties of solutions based on alkylene glycols. During long-term thermostatting at temperatures below minus 20°C, a sharp increase in viscosity occurs, sedimentation occurs, and solidification of ACHSPK solutions containing these components occurs.
The specified properties of ShchSPK and solutions based on ShchSPK lead to restrictions on the use of these products in the temperature range below minus 20°C (transportation and storage of ShchSPK and ShchSPK solutions), and also complicate the technology of their use (for example, spraying on surfaces or materials through jet devices and nozzle spraying), the uniformity of the coating also decreases.
Disclosure of the Invention
The technical result provided by the invention is to expand the arsenal of products based on alkaline waste from the production of caprolactam (ShSPK), intended for use at low temperatures (below 0°C), to create a technologically simple and inexpensive method for modifying ShchSPK for use as an independent product or in the composition of solutions and mixtures used at low temperatures (below 0°C), and the creation of a product (solution or mixture) with high performance characteristics: low pour point down to minus 35-70°C and at the same time low viscosity during long-term operation at low temperatures and stability of properties under conditions of prolonged exposure to low temperatures.
The technical result is achieved by a method of modifying the alkaline wastewater of caprolactam production by treating it with an acid or a mixture of acids or an aqueous solution of an acid or a mixture thereof to a pH value of 4-9, preferably to a pH value of 5-7.
The acid used is an organic acid, an inorganic acid, a mixture of organic acids, a mixture of inorganic acids, a mixture of organic and inorganic acids.
Preferably, acetic acid, citric acid, and formic acid are used as organic acid.
Preferably, hydrochloric acid, sulfuric acid, and perchloric acid are used as inorganic acid.
As an aqueous acid solution, use a 2-99% solution of an inorganic acid or a mixture thereof, a 2-99% solution of a monobasic carboxylic acid or a mixture thereof, a 2-99% solution of a C 2 -C 3 dibasic carboxylic acid or a mixture thereof, 5-99% solution of dibasic C 4 carboxylic acid, 10-99% solution of dibasic C 5 carboxylic acid, 20-99% solution of dibasic C 6 carboxylic acid, 2-99% solution of dibasic C 7 -C 18 carboxylic acid or their mixture, 2-99% solution of polybasic carboxylic acid or a mixture thereof.
The technical result is achieved in a solution for use at low temperatures, including ASPK, modified by treating it with an acid or a mixture of acids or an aqueous solution of an acid or a mixture thereof to a pH value of 4-9, preferably to a pH value of 5-7.
The solution for use may additionally contain an additive that lowers the pour point in an amount of 2-30% wt.
The degree of treatment of ACHSPK is fixed by changing the pH value of the solution:
at a pH of the solution of 13-10 (untreated ShchSPK), an increase in viscosity, a decrease in fluidity, precipitation and solidification of the solution occurs when thermostatted to minus 10-15°C;
at a solution pH of 9-8 (adding approximately 1-5% acid), an increase in viscosity, precipitation and solidification of the solution occurs when thermostatted to minus 30°C;
At a pH of the solution of 7-5 (adding approximately 3-8% acid), there is no increase in viscosity or precipitation; the solution solidifies when thermostatted to -35-45°C;
When the pH of the solution is 4-2 (adding more than 50% acid), there is also no increase in viscosity and no precipitation; the solution solidifies when thermostatted to -35°C, but such a solution has an acidic reaction; with a significant increase in acid concentration, the pour point of the solution increases , the solution is aggressive, corrosive.
Thus, the optimal pH value is 5-7 (neutral pH), which, among other things, reduces the corrosive effect on metals.
If a higher pH is required for the use of the product, its value after modification can be increased with compounds that have an alkaline reaction.
With an increase in alkalinity (increase in pH) of the modified ShchSPK solution, there is no longer an increase in viscosity, precipitation and an increase in the pour point, that is, the properties of the modified ShchSPK change irreversibly.
Modified ShchSPK can be used as an independent product or as part of solutions and mixtures.
Introduction into the solution of the modified ShchSPK additives that lower the pour point of aqueous solutions in an amount of 2-30% wt. additionally reduces the viscosity of the solution at low temperatures and lowers the pour point to minus 35-70°C.
As an additive that lowers the pour point, monohydric alcohol, and/or a mixture of monohydric alcohols, and/or polyhydric alcohol, and/or a mixture of polyhydric alcohols, and/or alkylene glycol, and/or a mixture of alkylene glycols, and/or alkylene glycol ether, and /or a mixture of alkylene glycol ethers, and/or an organic alkali metal acid salt, and/or a mixture of alkali metal organic acid salts, and/or an alkali metal inorganic acid salt, and/or a mixture of alkali metal inorganic acid salts.
Carrying out the invention
Modification of ShchSPK (for example, produced by OJSC KuibyshevAzot or OJSC KemerovoAzot) is carried out as follows.
ACHSPK is pumped into the reactor using a pump from a storage tank, and the required amount of acid (or acid solution) is determined at a rate of approximately 1-8% by weight. After introducing acid into the ACHSPK through the filler neck of the reactor, this composition is mixed to carry out modification. The degree of completion of the modification reaction is determined by changing the pH of the solution. Upon completion of the modification, the ShchSPK is poured into a container for the finished product.
The preparation of a solution based on modified ShchSPK with additives that lower the pour point is carried out as follows.
After the completion of the reaction of modifying the ChSPC, an additive in the amount of 2-30 wt.% is fed through the filler neck of the reactor, the composition is mixed until homogeneous. The resulting composition is poured into a container for the finished product.
Examples of implementation of the invention
In the examples given in Table 1, ShchSPK produced by OJSC Kuibyshevazot was used.
1. ShchSPK is pre-cooled in a cryostat bath at -20°C for about 3 hours. An increase in the viscosity of the ACHSPK solution and a limitation in the mobility of the solution (solidification) are observed.
2. ShchSPK with initial pH=10 is poured into the reactor. 1-8% acid or acid solution is added to the total mass of ShchSPK, the composition is stirred for about 30 minutes, the optimal temperature of the composition is 20°C. Hydrogen indicator pH=4-9.
3. Control measurement: for 3 hours the treated ASPK is cooled in a cryostat bath at -20°C, the solution remains mobile (does not harden).
The resulting modified ShchSPK is an easily mobile, homogeneous, stable liquid of dark brown color without sediment, which has a lower viscosity at low temperatures (below 0°C) and a lower pour point during long-term thermostatting (up to minus 35-45°C), and the use modified ShchSPK as an independent product or as part of solutions used at low temperatures (below 0°C), such as antifreezes, deicers, anti-freezing, freezing, sticking, dusting and blowing agents, anti-caking agents, preventive lubricants, etc., will significantly improve their performance characteristics at low temperatures.
4. To prepare a solution based on modified ShchSPK with additives that lower the pour point, after completion of the modification reaction, an additive in the amount of 2-30 wt.% is added to the total mass of the modified ShchSPK through the filler neck of the reactor, the solution is stirred until homogeneous for about 30 minutes. The resulting solution is poured into a container for the finished product.
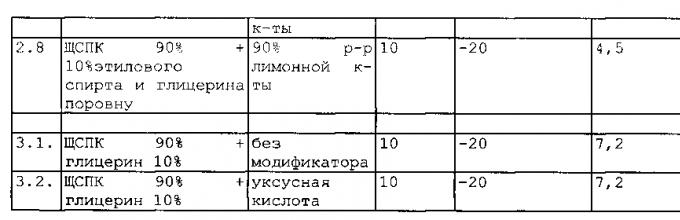
Test results of unmodified ShchSPK (example 1.1) and solutions based on unmodified ShchSPK (examples 2.1, 3.1, 4.1, 5.1, 6.1, 7.1, 8.1 and 9.1), modified ShchSPK (examples 1.2-1.7) and solutions based on modified ShchSPK (examples 2.2 -2.8, 3.2-3.8, 4.2-4.10, 5.2-5.7, 6.2-6.7, 7.2-7.6, 8.2-8.5, 9.2-9.5), as well as ShchSPK processed according to the prototype, are shown in Table 1.





1. A method for modifying alkaline wastewater from caprolactam production, characterized in that the alkaline wastewater is treated with an acid or a mixture of acids, or an aqueous solution of an acid, or a mixture thereof to a pH value of 4-9.
2. The method according to claim 1, characterized in that the alkaline wastewater is preferably treated to a pH value of 5-7.
3. The method according to claim 1, characterized in that the acid used is an organic acid, an inorganic acid, a mixture of organic acids, a mixture of inorganic acids, a mixture of organic and inorganic acids.
4. The method according to claim 3, characterized in that acetic acid, citric acid, formic acid is preferably used as an organic acid.
5. The method according to claim 3, characterized in that hydrochloric acid, sulfuric acid, perchloric acid is preferably used as an inorganic acid.
6. The method according to claim 1, characterized in that a 2-99% solution of an inorganic acid or a mixture thereof, a 2-99% solution of a monobasic carboxylic acid or a mixture thereof, a 2-99% solution is used as an aqueous acid solution C 2 -C 3 dibasic carboxylic acid or a mixture thereof, 5-99% solution of dibasic C 4 carboxylic acid, 10-99% solution of dibasic C 5 carboxylic acid, 20-99% solution of dibasic C 6 carboxylic acid, 2-99% - a dibasic C 7 -C 18 carboxylic acid solution or a mixture thereof, a 2-99% solution of a polybasic carboxylic acid or a mixture thereof.
7. A solution for use at low temperatures, characterized in that it includes an alkaline wastewater from the production of caprolactam, modified by the method according to claim 1.
8. The solution according to claim 7, characterized in that it additionally contains an additive that further reduces the pour point in an amount of 2-30% wt.
9. The solution according to claim 8, characterized in that the additive is monohydric alcohol, and/or a mixture of monohydric alcohols, and/or polyhydric alcohol, and/or a mixture of polyhydric alcohols, and/or alkylene glycol, and/or a mixture of alkylene glycols, and/or an alkylene glycol ether, and/or a mixture of alkylene glycol ethers, and/or an organic alkali metal acid salt, and/or a mixture of alkali metal organic acid salts, and/or an inorganic alkali metal acid salt, and/or a mixture of alkali metal inorganic acid salts .
Similar patents:
The invention relates to the field of geocryology, in particular to methods for producing anti-icing reagents that have various applications, the main of which is the use for preventing and removing ice on runways and taxiways of airfields in various weather and climatic conditions.
The invention relates to the field of public utilities and road service, in particular to liquid anti-icing compositions. The anti-icing composition contains, in wt.%: monohydric alcohol 1.0-10.0; Surfactant 0.10-0.30; corrosion inhibitor 0.5-1.0; if necessary, a thickener up to 4.0 and an aqueous solution of a carboxylic acid salt at a concentration of 15-60 formate and/or sodium or potassium acetate in terms of dry matter up to 100.
The invention relates to the coal mining industry to combat the freezing of coal, overburden rocks and their freezing to steel walls during transportation and storage.
The invention relates to methods for suppressing or reducing icing or snow formation on a surface using anti-icing compounds. The anti-icing liquid contains potassium acetate, water and an anti-corrosion additive, including sodium benzoate, and it additionally contains propylene glycol, and the anti-corrosion additive additionally contains benzotriazole, sodium dihydrogen phosphate, sodium tetraborate, diethanolamide based on sunflower oil acids, diethanolamine, and a cationic organosilicon surfactant.
The invention relates to methods for suppressing or reducing icing or snow formation on a surface using anti-icing compositions. Anti-icing liquid for airport runways contains potassium acetate, water and an anti-corrosion additive, including sodium benzoate, while it additionally contains sodium acetate and propylene glycol, and the anti-corrosion additive additionally contains benzotriazole, sodium dihydrogen phosphate, sodium tetraborate, diethanolamide based on sunflower oil acids , diethanolamine, cationic organosilicon surfactant.
The invention relates to methods for suppressing or reducing icing or snow formation on a surface using anti-icing compounds. The anti-icing liquid for airport runways contains potassium acetate, water and an anti-corrosion additive, including sodium benzoate, and it additionally contains potassium formate, and the anti-corrosion additive additionally contains benzotriazole, sodium dihydrogen phosphate, sodium tetraborate, diethanolamide based on sunflower oil acids, diethanolamine , an organosilicon surfactant of the cationic type. As a result, it is possible to increase environmental safety and reduce the corrosive activity of the deicing fluid.
The invention relates to compositions of household chemicals used for hydrophobization of products made from natural smooth and pile leather, and their protection from the negative effects of electrolyte solutions.
The present invention relates to the field of coating compositions, and more specifically relates to a coating composition comprising an amine hardener composition containing a bis-aromatic secondary diamine, a bis-aromatic primary diamine and optionally a mono-aromatic primary diamine.
Anti-ice reagent can be used to combat ice on roads, bridges, overpasses, and airfield runways. The anti-icing composition is prepared by mixing dolomite, hydrochloric and/or acetic acid and water, followed by the addition of a corrosion inhibitor. As an inhibitor, take the interaction product (IP) of 1 mole of a fatty amine, 10-30 moles of oxyethylene and 2 moles of a phosphorus-containing compound or a composition containing in wt.%: 5-50 higher fatty acids, 3-20 PV or a mixture of PV with an ethoxylated amine (OEA) with a degree of oxyethylation of 10-30 and a number of carbon atoms C8-C20, 3-20 nonionic surfactant (NSAS) and the rest is an organic solvent. The invention provides an anti-icing reagent with high melting ability, low corrosiveness and low pour point. 2 salary f-ly, 24 ave., 3 tables.
The anti-icing composition can be used to remove ice from airfield runways, highways, sidewalks, and other areas. The anti-icing composition includes calcium chloride, water and a reaction product (IP) of 1 mole of a fatty amine, 10-30 moles of ethylene oxide and 2 moles of a phosphorus-containing compound or a composition containing in wt.%: 5-50% higher fatty acids; 3-20% PV or a mixture of PV with ethoxylated amine (OEA) with the number of carbon atoms C8-C20 and degree of ethoxylation 10-30; 3-20% nonionic surfactant (NSAS); the rest is solvent. The anti-icing composition has a high melting ability, low corrosiveness and has a pour point of up to -60°C, and the method for its preparation is simple and economical. 2 salary f-ly, 31 ave., 3 tables.
The inventions relate to the field of chemistry, namely to polymer paints and varnishes that form a superhydrophobic coating on the protected surface after drying, and a method for producing a superhydrophobic coating for use to protect various structures and structures of construction, transport and energy, operated in conditions of open climatic precipitation in the form rain, snow, fog, icing, corrosion. The technical result of the inventions is to create a composition and method for producing a superhydrophobic coating with improved physical and mechanical characteristics and high anti-icing properties. The composition of the superhydrophobic coating includes, as a hydrophobic film former, a liquid hydrophobic polymer film former based on fluorourethane enamel "Viniftor", a hydrophobic material in the form of a powder mixture of micro- and nanoparticles of micron fluoroplastic 4 "Fluralit" with silanes modified nanodispersed silicon dioxide Aerosil R-812, taken at the ratio 20:1, hardener “Desmodur 75” and solvent o-xylene, with the following ratios of ingredients, wt. parts: hydrophobic film former - 100, hydrophobic material in the form of a powder mixture - 10-50, hardener "Desmodur 75" - 13, o-xylene solvent - 10. In the method for producing a superhydrophobic coating, a powder component is first prepared by intensive mixing of micro- and nanoparticles micron fluoroplastic 4 “Fluralit” with nanodispersed silicon dioxide Aerosil R-812. Then the liquid hydrophobic polymer film former based on fluorourethane enamel “Viniftor” is mixed with the hardener “Desmodur 75” and the mixture is adjusted to the specified viscosity by adding o-xylene solvent to it. The resulting hydrophobic material is applied by pneumatic spraying to the protected surface, and then a pre-prepared powder component is applied by electrostatic spraying to the uncured surface of the hydrophobic layer. After curing, a superhydrophobic coating is obtained, characterized by a contact angle of at least 153° and a coating service life of at least 10 years. 2 n.p. files, 2 tables, 4 pr.
The invention relates to a technology for producing anti-icing fluids (AFLs) intended to combat ground icing of aircraft. A method for preparing an anti-icing liquid involves preparing a concentrate by adding, with stirring, a surfactant based on fatty alcohols and a thickener based on polyacrylic acid to a water-glycolic or water-glycerin mixture used as a solvent, taken in an amount of 1-20 wt.% of its total required quantity. The resulting concentrate is added with stirring to the remaining part of the solvent, followed by stirring. Then, with stirring, a neutralizing agent, potassium hydroxide, is added to the resulting homogeneous suspension, followed by stirring. Mixing is carried out in a mixer with a stirrer. After mixing is completed, the resulting anti-icing liquid is degassed by discharging it from the mixer through an ultrasonic flow-through dispersant. The result is an increase in the stability of the operational characteristics of the anti-icing fluid during its storage before use. 1 ill., 3 pr., 3 tab.
The invention relates to the chemical industry, namely to the production of solid deicing materials with reduced corrosion activity based on table salt, calcined calcium chloride, and corrosion inhibitors. The invention describes five variants of de-icing material. The method for producing solid deicing material includes uniform mechanical mixing of first grade crystalline table salt, first grade crystalline technical calcined calcium chloride, crystal elements of a metal corrosion inhibitor, crystalline surfactant, and crystalline acidity regulator. In the process of producing deicing material, each element of the corrosion inhibitor is saturated with heavy 13C carbon isotopes in such a way that the ratio of the number of 13C carbon isotopes to the total amount of carbon in the element is from 0.005 to 0.75. Also, each element of the corrosion inhibitor is saturated with heavy nitrogen isotopes 15N such that the ratio of the number of nitrogen isotopes 15N to the total amount of nitrogen in the element is from 0.0001 to 0.1375. The technical result consists in reducing the corrosive activity of the deicing material due to reduced corrosion activity and increased efficiency of the corrosion inhibitor in the composition of the resulting solid deicing material as a result of enriching the corrosion inhibitor with heavy isotopes of carbon 13C and nitrogen 15N in a reactor installation with a cavitation reactor. 5 n.p. files, 4 ill., 68 tables.
The invention relates to the chemical industry, namely to solid deicing materials (variants) with reduced corrosive activity based on table salt, calcined calcium chloride, and corrosion inhibitors. The method for producing solid deicing material includes uniform mechanical mixing of first grade crystalline table salt, first grade crystalline technical calcined calcium chloride, crystal elements of a metal corrosion inhibitor, crystalline surfactant, and crystalline acidity regulator. In the process of producing deicing material, each element of the corrosion inhibitor is saturated with heavy isotopes of carbon 13C in such a way that the ratio of the number of carbon isotopes 13C to the total amount of carbon in the element is from 0.005 to 0.75, and also each element of the corrosion inhibitor is saturated with heavy isotopes of nitrogen 15N in such a way that that the ratio of the number of nitrogen isotopes 15N to the total amount of nitrogen in the element ranges from 0.0001 to 0.1375. The technical result achieved by the invention is to increase the effectiveness of the corrosion inhibitor in the composition of the resulting solid deicing material with reduced corrosive activity due to the enrichment of the corrosion inhibitor with heavy isotopes of carbon 13C and nitrogen 15N in a reactor installation with a cavitation reactor. 5 n.p. files, 4 ill., 68 tables.
The invention relates to the chemical industry, namely to deicing materials. The method for producing solid deicing material involves uniform mechanical mixing of crystalline food grade rock salt, crystalline calcium chloride, crystalline metal corrosion inhibitor elements, crystalline surfactant and crystalline acidity regulator. In the process of producing deicing material, each element of the corrosion inhibitor is saturated with heavy 13C carbon isotopes in such a way that the ratio of the number of 13C carbon isotopes to the total amount of carbon in the element is from 0.005 to 0.75. Also, each element of the corrosion inhibitor is saturated with heavy nitrogen isotopes 15N such that the ratio of the number of nitrogen isotopes 15N to the total amount of nitrogen in the element is from 0.0001 to 0.1375. EFFECT: increased efficiency of the corrosion inhibitor without compromising the deicing properties of the resulting solid deicing material. 5 n.p. f-ly, 4 ill., 69 tab.
The method can be used to reduce icing of a substrate, for example, wind generator blades. Apply to the substrate curable film-forming compositions containing a curing agent with isocyanate functional groups, and a film-forming polymer with functional groups reactive with respect to the isocyanate groups of the curing agent, and polysiloxane present in the curable film-forming composition in an amount sufficient to reduce icing of the substrate when exposed to conditions that promote ice formation. The polysiloxane contains polydimethylsiloxane and at least two hydroxyl and/or amino functional groups, or the polysiloxane contains at least one polysiloxane containing at least one functional group that is reactive to the functional groups of at least , one other component of the curable film-forming composition, and at least one polysiloxane that is not reactive with functional groups of the other components of the curable film-forming composition. The film-forming compositions can be applied directly to the surface of the substrate or to a layer of primer and/or topcoat on the substrate. The technical result is to ensure, during curing, a maximum average load on the coated substrate of 450 N when testing for ice adhesion. 10 salary files, 2 tables.
Preventive lubricant refers to compositions to prevent freezing of bulk materials, in particular coal, and to combat dust formation; it can be used in coal, mining, metallurgical, construction and other industries during transportation at subzero temperatures. Preventive lubricant to prevent freezing of bulk substances contains a low-solidification base fraction and a component that dissolves it. It contains oil refining sludge (OP sludge) as a low-solidification base fraction, and the alcoholic fraction of caprolactam (CAF) as a dissolving component. The technical result of the proposed preventive lubricant to prevent freezing of bulk substances is to reduce the freezing of coal and its freezing to the walls of cars, reducing costs (material and labor costs) during its transportation and unloading, which is achieved by applying it to coal and the inner surface of railway cars. 5 ill., 3 tables.
The invention relates to the chemical industry, namely to deicing materials. The method for producing solid deicing material involves uniform mechanical mixing of crystalline food grade rock salt, crystalline calcium chloride, crystalline metal corrosion inhibitor elements, crystalline surfactant and crystalline acidity regulator. In the process of producing deicing material, each element of the corrosion inhibitor is saturated with heavy 13C carbon isotopes in such a way that the ratio of the number of 13C carbon isotopes to the total amount of carbon in the element is from 0.005 to 0.75. Also, each element of the corrosion inhibitor is saturated with heavy nitrogen isotopes 15N such that the ratio of the number of nitrogen isotopes 15N to the total amount of nitrogen in the element is from 0.0001 to 0.1375. EFFECT: increased efficiency of the corrosion inhibitor without compromising the deicing properties of the resulting solid deicing material. 5 n.p. files, 4 ill., 69 tables.
The invention relates to the modification of alkaline caprolactam production waste for use as an independent product or as part of solutions and mixtures used at low temperatures, for example, as: antifreeze, deicer, deicing agent, anti-freezing, sticking, dusting and blowing agent, anti-caking agent, preventive lubrication, etc. The method of modifying the alkaline wastewater of caprolactam production consists of treating it with an acid or a mixture thereof or an aqueous solution of an acid or a mixture thereof to a pH value of 4-9. The technical result is the creation of a technologically simple, inexpensive method for modifying ShchSPK, as well as a solution for use at low temperatures with high performance characteristics: low pour point down to minus 35-70 ° C and low viscosity during long-term operation at low temperatures and stability of properties under long-term conditions exposure to low temperatures. 2 n. and 7 salary files, 1 table.
Plasticizing and air-entraining additive for
construction cement mortars and concretes. It is used as a component of cement mixtures to improve the technological performance of concrete and mortars in the construction of monolithic floors, floors, screeds, and in the manufacture of complex and critical monolithic structures and products.
Any cement mixture, be it mortar or concrete, requires mixing it with water. The actual water requirement of cement, i.e. amount of water
which it needs for hydration is about 15%.
However, there is another necessary requirement - the mobility of the mortar/concrete mixture. At a water-cement ratio (W/C = 15%) it will be
very rigid, practically “dry”: it cannot be laid or leveled, much less poured into the formwork.
To make the cement mixture mobile, about 30% water is added to it (W/C = 30%). When hardening such a solution or concrete, part of the water is spent on hydrating the cement, the rest - almost half -
evaporates or escapes through capillaries, leaving behind layers penetrated by communicating pores, causing additional shrinkage of concrete and cracks.
This is especially critical for structures with large linear dimensions, such as concrete screeds in floor structures or monolithic foundations. Through these pores, water gradually penetrates into the thickness of the concrete/mortar and, when frozen, destroys the structure and corrosion of the reinforcement occurs.
To reduce excess water, plasticizers are added to cement mixtures when stirring. These additives, by liquefying the concrete/mortar, make it mobile and almost “self-leveling” with a minimum of excess moisture.
Therefore, no excess water remains in the thickness of the concrete/mortar to be removed. No communicating pores are formed. Concrete acquires density, solidity, strength, its shrinkage is significantly reduced, and crack resistance increases.
The ShchSPK plasticizer, recommended for use in accordance with GOST 28013–89, has these advantages.
When mechanically mixing a cement mixture, ShchSPK promotes the inclusion of air microbubbles into the solution, which remain in it.
thicker in the form of closed spherical pores and further increase the crack resistance and bending strength of the structure.
ShchSPK increases the frost resistance of concrete by 1.5–2 times, reduces cement consumption to 8% while maintaining the required mobility and specified
strength.
MODE OF APPLICATION
ShchSPK is added to the mixing water or, with mechanical stirring, directly into the mixer. It is necessary to take into account: if you use ShchSPK, then to obtain the required mobility of the mixture you will need 20–30% less water than usual. If you use ShchSPK in plaster mortars, the best results are achieved in the top layers due to the creation of a dense, high-strength and water-resistant surface. If the concrete is prepared or transported by an automixer, you can add ShchSPK directly to the mixer in the amount of one package, about 5 liters or more, at the discretion of the master.
CONSUMPTION RATES
The optimal rate for introducing ASH into concrete/mortars is 0.3–1.2% by weight of cement, i.e. approximately 100–300 g per 100 kg of concrete/mortar. About adding ShchSPK to the mixer - see the end of the previous paragraph.
STORAGE
Shelf life 1 year. Storage temperature is unlimited.
After thawing, the physicochemical properties of ShchSPK are preserved. In case of slight separation during storage, stir before use.
SECURITY MEASURES
ShchSPK is a non-flammable liquid. Has an alkaline reaction. ACCORDING TO GOST 12.1.007–76, eating and smoking are prohibited in areas where ShchSPK is used. In case of contact with exposed skin, rinse quickly with water.
PACKAGE
Plastic bottle 5.25 l; 70 pieces per pallet.
ShchSPK- alkaline caprolactam production waste, which is a waste product of caprolactam production and is an aqueous solution of sodium salts of mono- and dicarboxylic acids, cyclohexanes and cyclahexanone. Brown liquid, moderately toxic, with a density at 20 ° C of 1.1 -1.2 g/cm 3, solution pH 10-13.
ShchSPK-m- modified alkaline caprolactam production waste, which is an aqueous solution of sodium salts of mono- and dicarboxylic acids, soda ash.
SPD-m- a product obtained on the basis of water-soluble high-boiling by-products of isoprene production. It is a highly mobile, non-separating liquid from yellow to brown.
NChK- an additive based on sodium or calcium salts of sulfonic acids, highly soluble in water. The liquid is dark brown in color, the density of a 10% aqueous solution is 1.023 g/cm3, 30% is 1.063 g/cm3.
KCHNR- an aqueous solution of neutralized acid tar. The liquid is dark brown in color, highly soluble in water, density 1.049 g/cm 3 .
GKZh-10-transparent liquid from pale yellow to brown, miscible with water in all proportions, density 1.19-1.21 g/cm3.
GKZh-11- a transparent liquid from pale yellow to brown, miscible with water in all proportions, density 1.19-1.21 g/cm3.
CHSS- a by-product of cellulose production, it is a solution of a complex mixture of organic and inorganic substances. Contains caustic soda, carbonate, sulfate, sodium thiosulfate and sodium sulfide, lignin and its destruction products, sugars and decomposition products of hemicelluloses, sodium salts of resin and fatty acids.
M 1 - sodium salts of water-insoluble organic acids. Supplied as a paste product with a dry matter content of at least 70% in metal or wooden barrels.
Air-entraining
FROM TO- saponified wood resin - a paste-like product based on the sodium salt of abietic acid, obtained by saponification of heat-treated wood resin with alkali. Low-toxic, fire- and explosion-proof. The release form is a slab in paper bags or a viscous product in barrels, transported by rail in covered wagons. Stored under a canopy or indoors in craft bags or barrels. Shelf life - 12 months.
START, START- neutralized air-entraining resin - additive based on sodium salts of abietic acid. Brown powder or monolith-block, the products are slowly soluble in water, low-toxic, low-flammable. Supplied in bags, in wooden or steel barrels with a capacity of 50 to 250 liters. Stored in enclosed areas to prevent the product from being moistened. The shelf life is unlimited.
The additive is introduced into the concrete mixture in the form of a 2...5% solution. The recommended dosage of the additive is 0.005...0.05% by weight of cement. When used as part of complex modifiers, SNV (to avoid coagulation) is administered separately from other additives.
The introduction of the additive helps to increase the tensile strength of concrete, increase crack resistance, gas and water resistance.
KTP- a mixture of derivatives of resin and fatty acids formed during the separation of tall oil from sulfate lignin. The solid product is brown in color and contains about 10% moisture. Let's dissolve well in water.
OTP- sodium salts of resin and fatty acids with a total alkalinity of 3-10%. Powder with a softening point of about 70°C.
OP- a white pasty product obtained by treating mono- and dialkylphenols with ethylene oxide, or an oily liquid from light yellow to light brown. Let's dissolve in water.
WITH- sulfonol refers to foaming additives, the foam ratio is 10 for a 1% aqueous solution, the surface tension is 20.9·10 -3 N/m, it is used in monolithic concrete and reinforced concrete structures with high frost resistance, lightweight porous concrete, mortars. WITH- synthetic soap, a mixture of sodium salts of alkylbenzenesulfonates C n H 2 n + 1 C 6 H 4 SO 3 Na, where n = 12,.. 18. White or light yellow powder, highly soluble in water. Non-toxic (irritates the upper respiratory tract). Release form - powder in bags or 45% solution. Supplied by rail in polyethylene or paper bags, in liquid form in tanks.
Gas-forming
GKZh-94- a polymer of ethylhydrosiloxane formed by the hydrolysis of ethyldichlorosilane. Active hydrogen content 1.3 – 1.42%. When using additives, the temperature of the concrete mixture should not exceed 30°C. Electrical heating of concrete is not allowed.
GKZh-94M- the same, with an active hydrogen content of 1.76%.
PGEN- a transparent mobile liquid, insoluble in water, forms an emulsion. The kinematic viscosity of a 50% solution in toluene at 20°C is 1.6-2.2 s; it is not recommended for heat treatment of concrete.
136-41(GKZh-94) and 136-157(GKZh-94m)– organosilicon liquids (oil) polyhydrosilaxanes, formed during the hydrolysis of ethyldichlorosilane, are colorless or light yellow non-toxic, explosive, flammable, water-insoluble liquids with a guaranteed shelf life of up to 1 year from the date of manufacture at temperatures from 0 up to 20°C. Under atmospheric influence, liquids can polymerize over time, turning into a jelly-like irreversible product.
Additives based on polyhydrosiloxanes are used in the form of emulsions. The preparation of emulsions is a rather complex process, so it is most reliable to use emulsions prepared directly by the manufacturer of the original product, because the manufacturer can select the most effective stabilizer to obtain a stable emulsion. Organosilicon emulsions may have different trade names from different manufacturers; technical parameters are indicated in the product passport. Organosilicon liquids and emulsions based on them have a hydrophobic (water-repellent) property, reducing the wettability of the material with water. On the one hand, when hydrogen is released in an alkaline environment, additional cohesion of polysiloxane chains occurs. These new formations, insoluble in water and solutions of inorganic substances, are deposited in micropores and capillaries and, to a certain extent, hinder the penetration of aggressive liquids into them. On the other hand, the resulting organometalcalcium siloxanes and silicon polymers of new chains with a trivalent bond between Si atoms, chemically fixing on the surface of the cement stone, hydrophobize the walls of pores and capillaries due to the formation of a hydrophobic film. This increases the resistance of concrete in various environments, since the adhesion of salt and ice crystals to the hydrophobic surface of the pores is reduced. Such additives are indispensable for concrete with high requirements for frost and salt resistance, regardless of its composition and type of binder, including at low temperatures (down to minus 60°C); for structures operated in aggressive environments, sea water.
KE-30-04- emulsion GKZh-94 in water - a homogeneous white liquid is supplied at a 50% concentration in a sealed container with a capacity of 20... 200 liters with a guaranteed shelf life of 6 months from the date of manufacture at a positive temperature not higher than 20°C. It is transported by all types of transport, ensuring the safety of the container from mechanical damage, precipitation and direct sunlight.
The emulsion is introduced into the concrete mixture with mixing water diluted to 10...25% or 50% concentration, depending on the capabilities of the dosing devices. Before use, the product is thoroughly mixed. Recommended dosages: GKZh-94-0.003... 0.1%, GKZh -94m -0.01... 0.07% by weight of cement in terms of 100% liquid. The effectiveness of additives increases with increasing mobility of the mixture and when using pozzolanic and slag Portland cement. The temperature of the prepared concrete mixture with additives should not exceed 30°C, therefore electrical heating of the concrete should be excluded.
PACK- aluminum powder, a silvery fine powder, soluble in acids and alkali solutions, but insoluble in water and organic solvents, is an effective gas generator for the production of aerated concrete. It is extremely fire hazardous. The powder is packaged in metal hermetically sealed jars with a capacity of 50 liters and stored in the manufacturer’s packaging in dry, closed rooms at a temperature not exceeding +35°C. They are transported by all types of covered transport with the installation of cans according to the principle of dense packaging, which prevents their movement.
Powder is introduced into the concrete mixture in the form of a specially prepared paste (see “Guide to the production and use of aluminum paste as a gas-forming agent for cellular concrete”, M., NIIZhB, 1977). The calculated amount of aluminum paste with a surfactant is introduced into the concrete mixture with mixing water. Recommended dosage is 0.005...0.01% by weight of the binder. The action of the additive is accompanied by the release of hydrogen. Overdose may reduce the strength of concrete. Preparation
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
Posted on http://www.allbest.ru/
Waste-free technologies based on caprolactam production waste
PRODUCTION OF AMMONIUM SULPHATE FROM CAPROLACTAM PRODUCTION WASTE
waste caprolactam construction inhibitor
Through the famous Beckmann rearrangement, cyclohexanone oxime is converted to caprolactam, a monomer for the production of nylon-6. In industrial practice, after rearrangement is complete, the reaction mixture is neutralized and the lactam is isolated from the mixture by extraction or other suitable methods. The most commonly used neutralizing agent is ammonium hydroxide. In this case, when sulfuric acid is used as a rearrangement catalyst, the by-product is ammonium sulfate, which cannot be reused in the production process. Ammonium sulfate can be sold as a fertilizer, but the product is usually available in sufficient quantities and is of low price.
In addition, for 1 ton of caprolactam produced, 3 tons of ammonium sulfate are formed, which creates problems with its disposal, since caprolactam production is constantly growing and prices for the by-product are low. The neutralization process consumes large amounts of water; it is exothermic and the heat generated is removed in the form of hot water and steam to maintain the temperature of the process. Large volumes of the reaction mass at the neutralization stage cause the high cost of separating lactam from the by-product and obtaining ammonium sulfate.
Neutralization of sulfuric acid with other bases results in the formation of even cheaper or less commonly used products. For example, calcium hydroxide, a cheap reagent, produces calcium sulfate at the neutralization stage, which has a low market price, is insoluble and prone to deposit formation and clogging of pipelines. Thus, the desirable alternative to existing production is not new methods for neutralizing and separating ammonium sulfate, but the development of a process that completely eliminates this problem.
A discussion on the production of caprolactam without the simultaneous formation of ammonium sulfate is presented by R. Mattone, G. Scioli and L. Gifre, Snia Viscose, see Hydrocarbon Processing *, January 1975.
See also US Pat. No. 4,015,946, Ammonium Sulfate from Acrylonitrile Wastewater, for a discussion of caprolactam waste treatment.
The invention relates to the building materials industry and can be used for the production of ceramic bricks, stones, blocks and tiles.
A known raw material mixture for the manufacture of wall ceramic products, including the following components, wt. clay shales from overburden of phosphorites 74-85; clay 10-25 and sulfate mixture waste from caprolactam production 1-5.
When firing bricks, sulfur dioxide, chlorine and vapors of corresponding acids are released from this raw material mixture, resulting from chemical reactions of Na 2 SO 4 and NaCl contained in the sulfate mixture with its other components. All these substances have a harmful effect on the human body, cause corrosion of technological equipment, do not allow the heat of exhaust gases to be utilized, for example, for drying raw bricks, and pollute the environment. Secondary sodium sulfate that has not decomposed, as well as the one formed during firing, is a water-soluble salt that forms efflorescence on the surface of the brick, reducing its durability and decorative properties. Sodium sulfate and carbonate contained in the sulfate mixture decompose at temperatures above 850 o C. The reactive sodium oxide formed as a result of this decomposition, which participates in the formation of neoplasms, interacts with the components of the clay (SiO 2, Al 2 O 3, FeO, etc. .) only after their amorphization, i.e. at a temperature above 900 o C. As a result, the firing temperature of the brick is 1000-1050 o C. In addition, brick from a known raw material mixture has an increased density and reduced strength, due to the presence of inert (non-reactive) , having a stable crystal lattice, silicon oxide (s-quartz), interacting with other oxide mixtures at temperatures above 1050 o C, and at a temperature of 1000-1050 o C it remains mainly in the form of inert inclusions and does not participate in the formation of durable ceramic shard.
A known raw material mixture for the manufacture of ceramic products contains active silica 72.4-74.7% thermal power plant ash 7.7-11.0% alkaline soap waste from chemical production 15.3-17.6% This mixture has significant disadvantages. The presence of sulfur compounds in the ash, and in most waste from soap production, for example, soap lyes up to 10% NaCl, causes the negative phenomena described above. The components included in the composition of alkaline soap-making waste do not provide the formation of polymerized particles of a colloidal composition of micelles, which promote the convergence of solid rock particles at the drying stage, increasing their reaction surface during the firing process. This factor, as well as the low content of active NaOH (0.1%) in the waste, which promotes the formation of the liquid phase, predetermines the occurrence of mainly solid-phase reactions during firing, which ultimately explains the relatively low compressive strength (268-305 kg/cm 2) products from this mixture fired at temperatures below 1100 o C. The need to conduct firing at temperatures above 1100 o C requires increased fuel costs, as well as costs for refractory materials for manufacturing and frequent repairs of the furnace and trolleys. The three-component composition of the mixture, in comparison with the two-component one, significantly complicates the production line and increases the cost of production.
A known raw material mixture for the manufacture of small-piece construction products, including, by weight. diatomaceous material 64-70; limestone 10-16; soap lye 16-25.
The disadvantages of this raw material mixture are: increased equipment costs and energy costs associated with the need to finely grind diatomaceous material and limestone (before passing through a 1 mm sieve) and the complexity of obtaining a homogeneous mixture of three components (the need to pass the mixture through a 1.5 mm sieve); high firing temperature of products (1100 o C) and their relatively low compressive strength (412-466 kg/cm 2) due to loosening of the structure of the semi-finished product by the released carbon dioxide and the occurrence of reactions in the solid phase; the formation of “dusts” and spalls in products from the contact of active CaO larger than 0.5 mm with atmospheric moisture (since limestone is ground to 1 mm, naturally the mixture contains particles larger than 0.5 mm, which, during firing, pass into the product) ; the release of chlorine during firing of products, the harmful effects of which have already been noted above. The closest to the recommended one is the raw material mixture for the manufacture of construction products, including, by weight. component from the group: tripoli, diatomite, opoka 66-72; calcium chloride production waste 6-12; soap lye 20-24.
The high content of chlorides and sulfates that are part of soap liquor and waste from the production of calcium chloride has a harmful effect on humans, equipment and product quality, as noted above. The release of a significant amount of gases (SO 2 , Cl, CO 2 , hydrocarbons) during firing of products leads to destruction of the continuity of the product, a shift in the sintering process to a temperature zone above 1000 (1120 o C) and a decrease in strength. The content of sulfates in the mixture does not allow the production of facial ceramic products from it due to fading and chipping on their surface. In addition, the increased content of carbonates and sulfates in the mixture causes the formation of gehlenite and anhydride in products, which also reduce the strength of products. The low (0.1%) content of free alkali in the soap liquor, the high content of calcium oxide in the mixture and the release of a large amount of gases from the products during firing predetermine the occurrence of reactions mainly in the solid phase. Sintering of the material occurs at high temperatures, which requires high fuel consumption and increases the cost of refractory materials for furnaces and trolleys. The strength of products from the mixtures specified in the prototype is also not very high for compression 498-510 kg/cm 2, and for bending 15.9-29.6 kg/cm 2.
The purpose of the invention is to reduce the firing temperature of ceramic wall products, increase their strength characteristics, utilize chemical production waste, and eliminate harmful emissions into the atmosphere.
This task is achieved by the fact that the raw material mixture for the manufacture of building bricks, including silica-containing raw materials and caprolactam production waste, contains amorphous-siliceous rock (opoku, diatomite, tripolite) as a silica-containing raw material, and alkaline caprolactam production waste as an alkaline waste. The use of amorphous-siliceous rock in an amount of 75-99 wt. together with alkaline waste from caprolactam production (ShchSPK) in an amount of 1-25 wt. ensures the production of a dense and durable structure of raw brick as a result of the interaction of amorphous silica, which is part of the amorphous-siliceous rock, with sodium salts of monodicarboxylic acids ShchSPK even during the drying process of the brick (100 o C) and the formation of polymerized particles of colloidal silica micelles enveloping solid particles contained in the rock, bringing them closer together and increasing the surface of reaction interaction during the firing process. The increased density of raw brick helps to prolong the process of burning out the organic substances of ShchSPK and its completion in the region of elevated temperatures. When burned, organic substances create a reducing environment and porous the material (product). Active NaOH, which is 20 times greater in ShchSPK (2.0% versus 0.1%) than in soap lye, and Na 2 O is a product of thermal dissociation of mono- and dicarboxylic acids of ShchSPK, interacts with amorphous silica to form alkali silicates: 2Na 2 O? SiO2? Na2? SiO 2 and Na 2 O? 2SiO2. The reducing environment and the proximity of amorphous silica particles, due to the formation of micelles, as well as the presence of other oxides (FeO, Al 2 O 3) in the mixture contribute to the formation of a highly active sodium silicate melt at a temperature of about 600 o C, which interacts with the solid phase, activating the process sintering of particles. As a result of crystallization of the melt, strong minerals are formed (albite, oligoclase, sodium ferrosilicate), which determine the high strength properties of products. When the mixture contains less than 1% ACHSPK, the formation of the melt shifts to the region of high temperatures (>800 o C). When the mixture contains more than 25% ACHSPK, an excessive amount of highly mobile (low viscosity) melt enriched in Na 2 O is formed, which, actively reacting with crystalline silicates, destroys the structural frame of the ceramic shard, reducing its strength. Thus, the use of the proposed mixture makes it possible to obtain At low firing temperatures, high-strength products with reduced density are produced, and the absence of harmful substances in the components of the mixture makes the process of producing products from the proposed mixture environmentally friendly and eliminates equipment corrosion.
For the manufacture of products, Kamyshlovsky diatomite, Balasheykinsky opoka, tripoli and ShchSPK containing sodium salts of organic acids 26.48 were used as raw material components of the mixture; resins 6.80; cyclohexanol 0.009; cyclohexanone 0.008; sodium hydroxide 2.0, water 64.703. The chemical compositions of diatomite, opoka and tripoli are given in table. 1. The production of samples is carried out as follows. Amorphous-siliceous rock (diatomaceous earth, opoku, tripolite) was crushed until passing through a sieve with a hole size of 3 mm, and then mixed with ShchSPK, which can be used in liquid form, in the form of a paste or dry form after dehydration at 100 o C, and also after pre-firing at 200-700 o C. Dried ASPK was also crushed to a particle size of less than 3 mm. After mixing the components, the mixture was moistened to 15% humidity and molded by semi-dry pressing at a pressure of 130 kg/cm2, sample cylinders with a diameter and height of 50 mm and plates 150 x 20 x 10 mm. Molding can also be carried out plastically, in this case the molding moisture content will be 30%. The samples were dried at 100 o C for 2 hours, and then fired at 680-1000 o C (depending on the content of ASHSPK in the mixture) with exposure at maximum temperature for 30 minutes. The rate of raising the firing temperature to the maximum was 10 degrees/min. The samples were cooled for 2-3 hours. Depending on the ratio of the components in the mixture and the firing temperature, the samples have a color from milky white to bright red.
When the firing temperature increases above the maximum, deformation or swelling of the samples is observed, and at a temperature below the minimum, their quality indicators sharply drop. Thus, the advantages of the proposed mixture over the mixtures given in the prototype (NN 10, 11, 12) and in analogues are as follows: firing temperature products from the proposed mixture are 300-400 o C lower, which guarantees a significant reduction in energy costs for the production of products, an increase in the service life of furnaces and trolleys, as well as a reduction in the cost of materials for their manufacture, since the need for refractories is reduced: with a lower density, and therefore, the mass strength of products from the proposed mixture is higher than that of products from the mixtures specified in the prototype and analogues; When products are fired, no harmful substances are released.
Raw mixture for the manufacture of construction products
Formula of invention: A raw material mixture for the manufacture of construction products, including a component from the tripol group, diatomaceous earth, flask and alkaline production waste, characterized in that as an alkaline waste it contains an alkaline waste from the production of caprolaklam in the following ratio of components, wt. Component from the tripol group, diatomite, flask 75 99 Alkaline waste from caprolactam production (dry) 1 25
The invention relates to the field of protecting metals from corrosion and can be used in the oil and gas industry, specifically to protect oil production equipment from acid corrosion, including hydrogen sulfide. The essence of the invention: the inhibitor contains oxygen-containing waste from the production of caprolactam, which uses a distillation cube of the products of cyclohexane oxidation and dehydrogenation of cyclohexanol or its mixture with the alcohol fraction of caprolactam production, and a nitrogen-containing additive, which contains monoethanolamine or nitrogen-containing waste from the production of ammonia or caprolactam in a mass ratio oxygen and nitrogen-containing component in a mixture of 2.5 - 1:1. 3 salary files, 1 table. The invention relates to the field of protecting metals from corrosion and can be used in the oil and gas industry, specifically to protect oil production equipment from acid corrosion, including hydrogen sulfide.
A large number of compositions of inhibitors of acid corrosion of metals, including nitrogen-, sulfur-, phosphorus-containing and unsaturated compounds, are known from the prior art.
Of these, corrosion inhibitors produced from waste from petrochemical production are of greatest practical interest. Involving production waste in the synthesis of inhibitors can significantly expand the raw material base, reduce costs, and also increase the efficiency of the main production.
A known atmospheric corrosion inhibitor is based on caprolactam production waste, namely the heavy fraction obtained after vacuum separation of cyclohexanone and cyclohexanol from the distillation residue of the by-products of cyclohexane oxidation and dehydrogenation of cyclohexanol (POD oil).
The disadvantages of the composition include its high efficiency as an inhibitor of acid corrosion in oil environments, and a large amount of waste when producing the inhibitor, since only part of the POD oil is used. The closest in technical essence to the invention is a composition of an inhibitor of acid corrosion in oilfield environments, containing waste from the production of caprolactam and a nitrogen-containing additive. Large volumes of consumption of acid corrosion inhibitors in the oil and gas and oil refining industries dictate the need to develop an inhibitor composition characterized by high protection efficiency, low production costs, and availability of raw materials.
This goal is achieved by the fact that the inhibitor of acid corrosion in oilfield environments contains oxygen-containing waste from the production of caprolactam and a nitrogen-containing organic additive, and these wastes contain a cube of rectification of the products of cyclohexane oxidation and dehydrogenation of cyclohexanol or its mixture with the alcohol fraction of caprolactam production, taken in a mass ratio of 4:1 , and as a nitrogen-containing additive - monoethanolamine or waste from ammonia production, or caprolactam at a mass ratio of oxygen and nitrogen-containing components in a mixture of 2.5-1: 1. In this case, the bottoms of monoethanolamine gas purification are used as nitrogen-containing waste from ammonia production, and ammonia production waste, the bottoms of monoethanolamine gas purification are used, and the bottoms of caprolactam distillation are used as caprolactam production wastes.
A comparative analysis with the composition of the prototype allows us to conclude that the proposed composition of the corrosion inhibitor differs from the known one by the introduction of new components, namely, as an oxygen-containing waste from the production of caprolactam, a cube of rectification of the products of oxidation and dehydrogenation of cyclohexanol (POD oil), a mixture with an organic solvent - the alcohol fraction, is used production of caprolactam (SPPC), taken in a mass ratio of 4:1. Monoethanolamine or nitrogen-containing waste from the production of ammonia (bottom residue from monoethanolamine gas purification) or caprolactam (bottom residue from caprolactam distillation) was used as a nitrogen-containing additive.
Thus, the proposed technical solution meets the “novelty” criterion.
An analysis of known compositions of acid corrosion inhibitors showed that some of the components introduced into the proposed formulation are known, however, their inhibitory functions are weakly expressed (see table, examples 2 and 3).
At the same time, special studies carried out in the latter case have proven that the anti-corrosion properties of POD oil as an individual component, as well as when it is mechanically introduced into the paint coating formulation, are practically not manifested. The protective properties of POD oil appear only when using a special technology for its introduction into the composition.
The components of the proposed formulation form a synergistic mixture that can significantly increase the effectiveness of corrosion protection in various oilfield environments. Thus, based on the above, we can conclude that the proposed solution meets the “inventive step” criterion. As a result of the implementation of the invention, the following technical and socio-economic effect is achieved. The proposed inhibitor provides high efficiency of corrosion protection in hydrocarbon, aqueous, and two-phase environments in a wide temperature range of use (from -40 to +60°C); the production of the inhibitor is based on the available raw material base, including waste from large-scale production that is not currently in qualified use. This makes it possible to significantly reduce the production cost of the inhibitor relative to well-known analogues (cheap raw materials, organization of production at the location of raw material sources, saving energy resources for waste disposal, etc.), and at the same time significantly improve the technical and economic efficiency of the main production (caprolactam); qualified use of the main large-scale waste from caprolactam production significantly improves the economic performance of the technology.
For experimental testing of the proposed inhibitor composition, 16 samples were prepared, 8 of which showed optimal results. The results are presented in the table of examples. As oxygen-containing waste from the production of caprolactam, we used “POD oil”, corresponding to TU 113-03-476-89 or its mixture with the alcohol fraction of caprolactam production (SFPK), corresponding to TU 113-03-10-5-85 . POD oil is a residue from the rectification of the products of cyclohexane oxidation and cyclohexanol dehydrogenation. The product contains esters of carboxylic acids, highly volatile components (low molecular alcohols and aldehydes), cyclohexanol, cyclohexanone, cyclohexylidene-cyclohexanol, heavy high-boiling polycondensation and polymerization products. The introduction of SFPK into the composition in the ratio oil POD: SFPK = 4:1, along with improving the effectiveness of protection, can significantly improve the performance characteristics of the formulation and expand the temperature range of its use (see examples 10 and 12).
As a nitrogen-containing organic additive, we used either monoethanolamine (TU 6-02-915-84), or nitrogen-containing waste from the production of ammonia or caprolactam, specifically the bottoms of the monoethanolamine gas purification of ammonia production (having the composition, wt.%: monoethanolamine 40-80, water 15 -50, impurities 5-15), which is currently being burned, or the bottom product of caprolactam distillation, corresponding to TU 113-03-10-6-84.
To reduce the viscosity of the inhibitor, the addition of a surfactant such as ethoxylated alkylphenols, for example OP-7 or OP-10, can also be added to its composition. The specified additive can be introduced into the composition in an amount of up to 5 wt.% by weight of the inhibitor.
The inhibitor is obtained by simply mixing the ingredients at a temperature of 20-60°C and a stirring time of 2-4 hours. The optimal concentration of the inhibitor in the water-oil emulsion is 50-200 mg/l.
Testing of the inhibitory properties of the proposed inhibitor was carried out according to the standard method (GOST 9.506-87, section 2 OST 14-15-15-7-85) with the following changes:
Flat samples (plates) of steel St. were used as control samples. 3 according to GOST 380-91, size 50x20x2 mm, with holes at one end with a diameter of 4 mm;
As a reaction medium, a highly mineralized oil field medium from the Kuibyshevneft Production Association was used, with the following characteristics: hydrogen sulfide content from 140 to 600.0 mg/l, pH 5.4-6.2, density 1.025-1.162 g/cm3, degree of mineralization 100 -250 g/l, as well as NaCE medium; hydrogen sulfide content 1156 mg/l, pH 3.35;
tests were carried out by gravimetric and electrochemical methods in dynamic mode;
Test duration is 6 hours at 20 and 60°C. The concentration of the inhibitor in the test stream was 50-200 mg/l. The component composition of the inhibitor and the results of corrosion tests of the prepared samples are presented in the table of examples. Examples 1-6 show the test results of a prototype inhibitor sample (example 1) and individual components of the proposed formulation (examples 2-6). As can be seen from the data presented, individual components exhibit a low protective effect. The highest degree of protection of 50.9-55.3% is achieved only in the case of using monoethanolamine or MEA bottom residue when their content in the flow is at least 200 mg/l. When the ratio of oil POD: nitrogen-containing component is below 1:1 (example 8), the protective effect is reduced; when it is above 1.5:1 (example 11), it does not increase by more than 85%. At an optimal ratio of POD oil: nitrogen-containing component of 1-2.5:1, a maximum protective effect of 87.8-100% is achieved at an inhibitor concentration of 50-200 mg/l (examples 7, 9, 10, 14, 15 and 16).
Examples 12 and 13 illustrate the improvement in performance characteristics (pour point and viscosity) with the introduction of SPFC and OP-7. Thus, it follows from the table that the components of the proposed formulation form a synergistic mixture, which makes it possible to significantly increase the protection efficiency in a mineralized coal-bearing stream, compared to inhibitory ability of individual components
ACID CORROSION INHIBITOR IN OILFIELD ENVIRONMENTS
An inhibitor of acid corrosion in oilfield environments, including oxygen-containing waste from caprolactam production and a nitrogen-containing organic additive, characterized in that as an oxygen-containing production waste it contains a cube of rectification of the products of cyclohexane oxidation and dehydrogenation of cyclohexanol or its mixture with the alcohol fraction of caprolactam production, and as a nitrogen-containing additive - monoethanolamine or nitrogen-containing waste from the production of ammonia or caprolactam with a mass ratio of oxygen- and nitrogen-containing components in the mixture of 2.5 - 1:1.
2. The inhibitor according to claim 1, characterized in that the bottoms of monoethanolamine gas purification are used as nitrogen-containing waste from ammonia production.
3. The inhibitor according to claim 1, characterized in that the bottoms of caprolactam distillation are used as nitrogen-containing waste from the production of caprolactam.
4. The inhibitor according to claim 1, characterized in that the mass ratio of the components in the mixture of the distillation cube of the products of cyclohexane oxidation and dehydrogenation of cyclohexanol and the alcohol fraction of caprolactam production is 4: 1.
Posted on Allbest
Similar documents
General characteristics of recycling and options for using waste from the metallurgical complex and chemical production in industry. The main directions of utilization of graphite dust. Evaluation of ash and slag waste as a raw material for construction materials.
abstract, added 05/27/2010
Current state of environmental safety problems in the field of waste recycling. Methods for processing radioactive, medical, industrial and biological waste. Thermal neutralization of toxic industrial waste.
abstract, added 05/26/2015
Types of household waste, recycling problem. Biological processing of industrial waste, dairy industry waste. Waste from the pulp and paper industry. Recycling waste after water purification. Sludge processing, waste biodegradation.
course work, added 11/13/2010
Features of recycling waste from the engineering complex, wood processing and production of building materials. Analysis of trends in the treatment of industrial waste at landfills of enterprises with factory technology for neutralization and disposal.
abstract, added 05/27/2010
Air and hydraulic classification of industrial waste according to the degree of danger to human health. Study of the design and operating principle of structures for mechanical preparation and processing of solid industrial waste.
presentation, added 12/17/2015
Environmental protection. Recycling of household waste and industrial waste. Waste-free technologies. Industrial recycling of solid household waste. Environmental monitoring. Monitoring students on methods of processing solid household waste.
abstract, added 01/14/2009
Methods for determining the hazard class of toxic production and consumption waste. Analysis of hazard indicators and concentrations of waste components. Temporary storage of production and consumption waste. Requirements for the placement and maintenance of objects.
test, added 05/13/2014
Special types of impact on the biosphere, pollution by industrial waste, protection from waste. Solid waste incineration: dioxin hazard, fees for storage and disposal of waste. Disposal of certain types of waste and fluorescent lamps, recycling.
course work, added 10/13/2009
The problem of waste disposal in the Ural cities. Investments and development plan for a plant for processing municipal solid waste (MSW). Interview with the Minister of Natural Resources. Problems of processing and disposal of industrial waste. Waste recycling methods.
abstract, added 11/02/2008
State of wastewater in the Baikal region. The influence of heavy metals on the environment and humans. Specifics of wastewater treatment based on waste. The global problem of recycling large-scale organochlorine and ash and slag waste, ways to solve it.